Serving The Life Science Industry
- Laser micro components (polymer, metal, ceramic, glass)
- Core wires, stylets, pushing wires, packaging, welding & forming mandrels
- Hypotubes
- Wire & tube components (coated/uncoated)
- OEM Balloons
- Thermoplastic & thermo set extrusions & injection molding
- Laser micromachining equipment for polymer, metal, ceramic & glass
- Catheter RF processing equipment (welding, tipping, flaring, hole punching)
- Micro-coiling and custom metal forming equipment
- Downstream & upstream extrusion equipment
- Measurement, vision & test equipment
- Integrated extrusion lines
- Feeding, on/off loading, cutting, complete cell integration
- (Semi-) Finished OEM catheters, IV & blood collection, Infusion, Drainage, neurology, cardiology
- Naso Gastric & Jejunal Feeding tube catheters & assemblies
- Endotracheal, Laryngeal mask, Airway management
- OEM guidewire torquers
- Stents, braided shafts, balloons
- All can come upon request: bulk, custom-packed, certified, sterilized.
- Conception
- Development
- Next generation selection
- Cost reduction
- Validation
- Certification
- Production start up for Catheters
- Closure/Delivery devices
- Guide Wires


Easytor® Guidewire Torquer
FDA 510 (k) application is in progress and pending. CE application in progress and pending.
The EASYTOR® is a single hand controlled guide wire torque – a unique solution.
It is developed primarily to give cardiologists performing procedures a handy and confident instrument.
It is easy to operate -enhancing accuracy and overall efficiency.
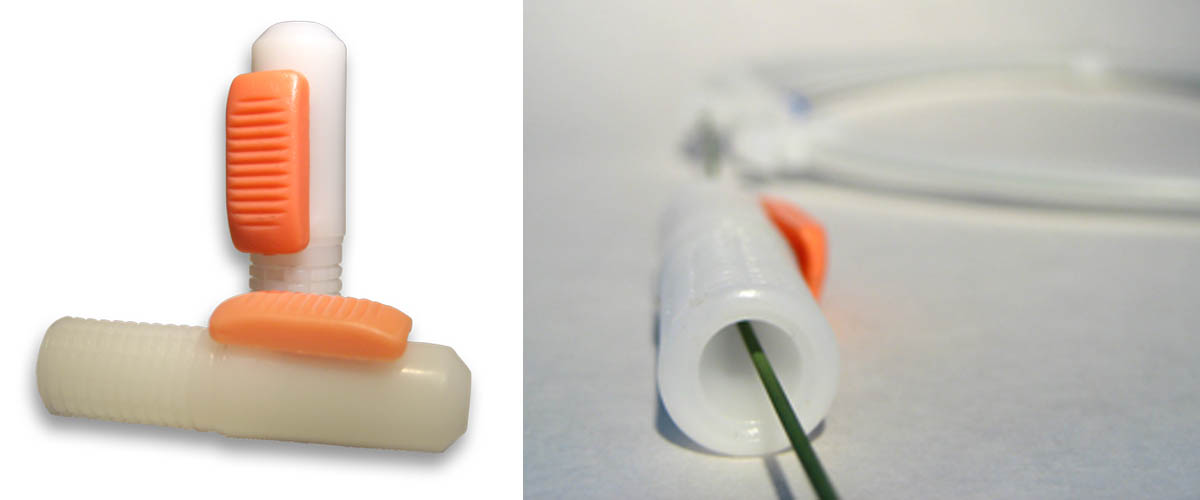
Main advantage: easy 1-hand operation
- No damage of plastic layer or surface coating
- No movement of guide wire when attaching and detaching
- Strong holding force during rotation of the guide wire
- Improved accuracy as cardiologists now can keep eyes on the monitor when attaching and detaching
- Low weight & ergonomic
- For all guide wire sizes 0.010″-0.038″ also PTFE coated
- Available in bulk unsterile and custom-packed, sterilized, CE certified
- Competitively priced!
For additional information please visit www.guidewiretorque.com with additional information, an instruction video and Tweets on this unique guide wire torque.


Our products are used in a wide range of medical specialties including the cardiovascular system, urology, stomatology and gastroenterology, neurosurgery, traumatology, gynecology and obstetrics, nephrology, oncology, pediatrics, neonatology and childcare, laboratory equipment and other applications.
9 Production facilities across the globe with 5 located in Europe, including HQ in Spain.
2Spring - visit address: J.F. Vlekkenweg 10-08 - 5026RJ - Tilburg - The Netherlands - Phone +(31) 13-5802420 - Fax +(31) 13-5443735 - Email: info@2spring.com
©2023 2Spring - iRuud.nl
